The Basic research Laboratory is located at the Parque Científico Barcelona (www.pcb.ub). Our main goal is to understand the cellular and molecular basis of human reproduction, in order to develop and optimize diagnostic and therapeutic procedures for patients. We address this main goal by studying what causes infertility, how to recognize quality in gametes and how to increase the rate of healthy pregnancies in fertility treatments. Our overall interests are in the areas of oocyte biology, sperm factors involved in fertilization, and implantation.
OOCYTE BIOLOGY
Role of lncRNAs in oocyte developmental competence (Project Leader: Dr. Montse Barragán)
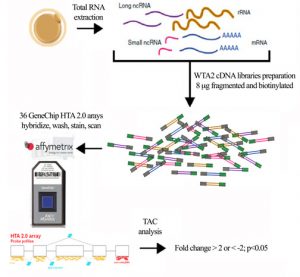
Oocyte quality diminishes with age. We are currently researching the relationship between oocyte quality and ovarian reserve. Our lab is focusing on the role of long non-coding RNAs in oocyte biology. In collaboration with Affymetrix (Thermo Fisher Scientific, USA) we have conducted single cell transcriptomic analyses of oocytes from women of different ages and levels of ovarian reserve, aiming to understand the role lncRNAs in oocyte maturation, fertility, and early embryo development. We have identified a number of lncRNAs which are differentially expressed in relation to age and/or ovarian reserve (Barragán et al. 2017). In particular, we are now focusing on the role of differentially expressed lncRNAs on regulation of acquisition of oocyte developmental competence. In collaboration with Prof. D. Grinberg from the University of Barcelona (www.ub.edu/genetica/humanaen/grinberg.htm) we are performing loss of function assays for selected lncRNA candidates of interest.
In vitro acquisition of competence (Project Leader: Dr. Anna Ferrer)
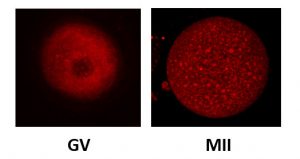
Upon follicular puncture, a stimulated ovary may yield oocytes at varying stages of maturation. In addition to mature MII oocytes, immature oocytes (arrested at the germinal vesicle (GV) stage), or at intermediate stages (MI) of maturation may be obtained.
When cultured in vitro, some MI oocytes can reach the MII stage, and we have shown that these in vitro matured MII oocytes can lead to successful pregnancies (Martin Palomino et al., manuscript in preparation).
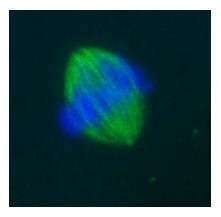
We are now systematically comparing MII oocytes matured in vitro using different culture conditions in terms of meiotic spindle morphology, chromosome arrangement, mitochondrial membrane potential, ER morphology, cortical granule distribution and cytoskeletal morphology to identify the optimal culture conditions that allow a proper nuclear and cytoplasmic maturation.
FERTILIZATION AND SPERM
Role of Phospholipase C zeta (PLCζ) in fertilization in fertilization (Project Leader: Dr. Montse Barragán)
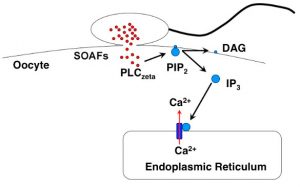
The PLCζ gene encodes a phospholipase C family member protein that hydrolyzes phosphatidylinositol 4,5-biphosphate, resulting in diacylglycerol and inositol 1,4,5-triphosphate. PLCζ is highly expressed in sperm and localizes to the acrosome. Upon fertilization, PLCζ is involved in triggering the Ca(2+) oscillations that initialize oocyte activation and subsequent embryo development. Specific levels of PLCζ are required for normal development. Sperm lacking PLCζ fail to induce Ca(2+) oscillations. We and others have described several PLCζ mutations associated to male infertility; we find that normozoospermic males can have disrupted expression and distribution of PLCζ, and reduced activation ability after ICSI in human oocytes (despite their reported normal activation potential when functionally tested using mouse oocytes), but that failed fertilization occurs even when levels and distribution of PLCζ protein are within normal range (Ferrer-Vaquer el al., J. Assist. Rep. Genet. 2016). Therefore, PLCζ seems to be a necessary but not sufficient factor in determining the molecular pathway involved in oocyte activation.
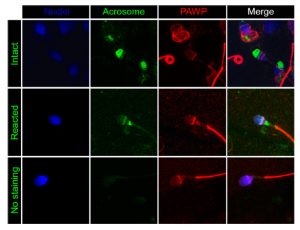
Role of PAWP in fertilization (Project Leader: Dr. Montse Barragán)
PAWP is a WW domain containing protein found in the post-acrosomal sheath region that is thought to induce calcium signaling, meiotic resumption and pronuclear development of MII arrested oocytes. These events appear mediated by a PPXY motif found in the C-terminal half of the protein. We have recently determined that PAWP mRNA and protein expression levels in sperm are not related to semen parameters, fertilization, or reproductive outcomes (Freour et al. J. Assist. Reprod. Genet. 2017). Nevertheless, a role for this protein in downstream events of fertilization cannot be ruled out and is the current focus of our laboratory.
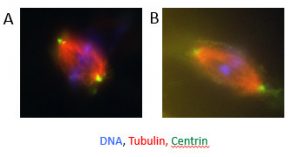
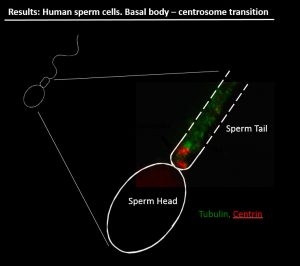
Role of the centrosome in early development (Project Leader: Dr. Rita Vassena)
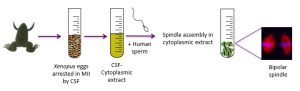
We are actively investigating the role of post-translational modifications of tubulin in sperm motility, as well as the role of sperm centrosomes in mitotic spindle formation. In collaboration with Prof. I. Vernos at the Center for Genomic Regulation, Barcelona (www.crg.eu), we have developed a model based on Xenopus oocyte cytoplasmic extracts. Interestingly, human sperm is capable of inducing microtubule nucleation and bipolar spindle formation in Xenopus oocyte cytoplasmic extracts, providing a convenient system to study the role of the human sperm centrosome in fertilization and early embryonic cell division. A central player in this phenomenon is the centrosome, a subcellular structure central to the formation of the spindle that is absent from the oocyte and provided to the zygote by the sperm. Our hypothesis is that centrosome defects may cause early developmental defects. When human sperm is added to Xenopus oocyte extract, bipolar spindles are formed with an efficiency of 70%. Furthermore, we are developing a technique to microinject isolated sperm tails into oocytes, allowing us to test the hypothesis that delivery of the centrosome itself (but not the sperm head) can improve embryo development after parthenogenetic activation.
IMPLANTATION
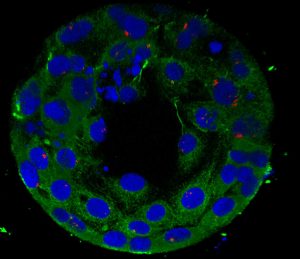
In vitro modeling of human implantation (Project Leader: Dr. Gustavo Tiscornia)
We are currently developing in vitro models of human implantation consisting of two elements: a) an element representing the embryo and b) an element representing the endometrium. The embryo may be conveniently represented by trophoblast spheroids obtained from trophoblast cell lines; the endometrial element can be constituted from endometrial epithelial cell lines or human endometrial stromal primary cultures.
In collaboration with Prof. J. Santaló from Universidad Autónoma de Barcelona (www.uab.cat) we aim to set up an in vitro model to mimic human implantation, and are using it to measure attachment and invasion rates of human embryos on endometrial cell layers derived from healthy women vs. patients with recurrent implantation failure (RIF), with the goal of measuring functional and molecular differences between endometrial samples.