OOCYTE BIOLOGY
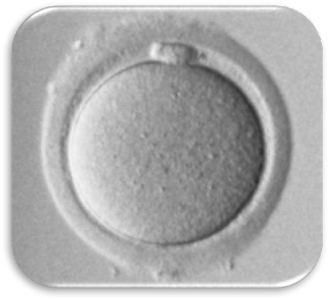
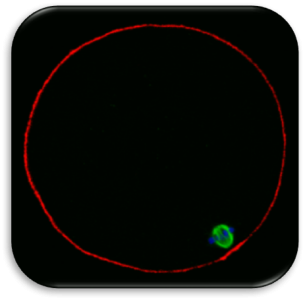
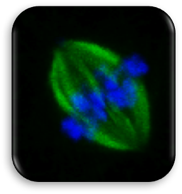
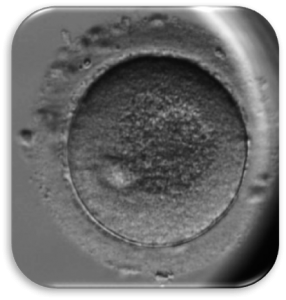
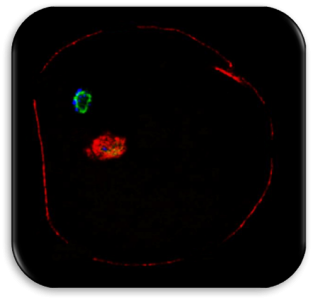
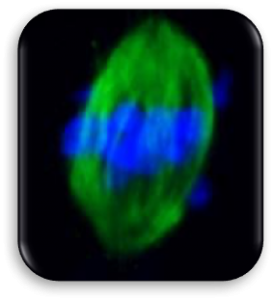
Impact of Dysmorphic patterns of human oocytes and embryo viability and success rate (Project Leader: Dr. Maria Beatrice Dal Canto) Recent research has described the ultrastructure of the healthy human oocyte and reveled a set of morphometric parameters that can be used to asses oocyte quality, such as the length of the spindle major axis, the length of the equatorial axis and the area of maximum projection. Specific morphological anomalies that may be observed in human oocytes can becytoskeletal damage, spindle disorganization, chromosome missalignment and cortical actin disorganization. These defects may might contribute to higher prevalence of aneuploidies in embryos. We have demonstrated that morphological anomalies in mature human oocytes, as revealed by transmitted light microscopy, correlate to intrinsic meiotic spindle actin cytoskeletal damage. Dysmorphic oocytes of all types are characterized by cortical actin meshwork discontinuities. This might have significant implications for embryo development. We are now focusing on the impact of morphological anomalies on fertilization process and embryo development.